Metabolism of fatty acids, ketone bodies.
Questions
01. Reaction of synthesis and utilization of ketone bodies. Biological role.
02. Mechanism
of ketosis in diabetes mellitus and starvation. Ketoacidosis.
03. Biosynthesis
of fatty acids:
3.1. Sources of acetyl CoA and NADPH I cytoplasm.
3.2. Synthesis of malonyl CoA.
3.3. Fatty acids synthesis, structure.
3.4. Biosynthesis of palmitic acid: reactions.
4. Biosynthesis
of triacylglycerols.
5. Biosynthesis
of phospholipids.
6. Fatty
infiltration of liver. Lipotropic agents.
01. Reaction of synthesis and utilization
of ketone bodies. Biological role.
Metabolism of ketone bodies
-
Fasting,
-
prolonged
physical exertion and cases when the cells do not get enough glucose (a diet
low in carbohydrates,
-
gastrointestinal
disorders,
-
glucosuria,
and diabetes mellitus)
activates
the breakdown of fat in adipose tissue.
Fatty
acids are
transported in the liver in a larger amount than usually which increases speed
of β-oxidation.
-
TCA cycle
activity is reduced in these conditions,
-
because
oxaloacetate is used in gluconeogenesis.
As a result,
the rate of acetyl CoA formation exceeds the ability of TCA cycle to
oxidize it.
Acetyl-CoA
accumulates in the mitochondria of the liver and is used for the
synthesis of acetoacetate.
-
This
substance may be released into the blood by the liver or converted to other
ketone body – β-hydroxybutyrate by reduction.
In hepatocyte with active β-oxidation, a high concentration of NADH occurs.
At high
concentrations of acetoacetate, its part is decarboxylated
non-enzymatically and turns into acetone.
-
Acetone
is not utilized by tissue, but is excreted in the urine and exhaled
air.
In this way
the body removes excess amount of ketone bodies, which do not have time to
oxidize and cause acidosis.
The rate of
synthesis of ketone bodies depends on the activity of 3-hydroxy-3-methylglutaryl-CoA
synthase (HMG-CoA synthase).
-
This
enzyme is inducible,
-
its
synthesis increases with increasing the concentration of fatty acids
in the blood.
-
HMG-CoA
synthase is inhibited by high concentrations of free CoA.
A small
amount of ketone bodies (their concentration in the blood of 10-30 mg/liter or
up to 0.2 mmol/l) is norm.
In the liver
acetoacetate cannot be oxidized,
-
so,
it flows with the blood into skeletal muscle, heart, brain, which is
capable of converting acetoacetic acid again to acetyl-CoA.
Content of ketone
bodies in the blood increases when the main source of energy for the body are
fatty acids - in the prolonged muscular work, starvation, diabetes
melitus.
Increase of
ketone bodies concentration in the blood is called ketonemia,
the
allocation of ketone bodies in the urine is called ketonuria.
Accumulation
of ketone bodies in the body leads to ketoacidosis:
-
alkali
reserve reduces, and in severe cases – a shift of pH occurs,
- as β-hydroxybutyrate and acetoacetate are water-soluble organic acids capable of dissociation.
Acidosis
reaches dangerous quantities in case of diabetes melitus.
The content
of ketone bodies in the blood in this disease increases 100 and more
times, achieving a concentration of 4-5 g/l.
Severe form
of acidosis is one of the main causes of death in diabetes melitus.
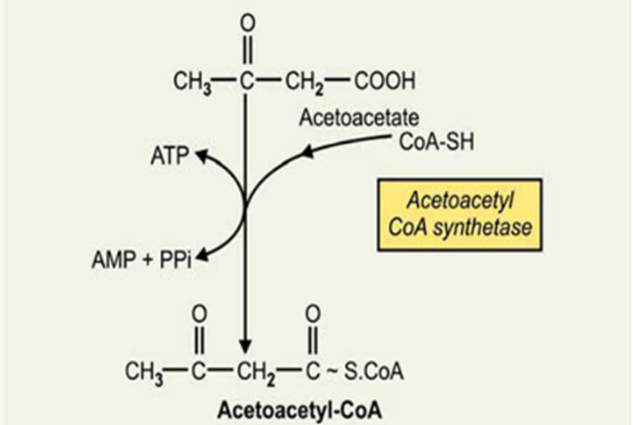
In
extrahepatic tissues acetoacetate is activated to acetoacetyl-CoA by succinyl-CoA-acetoacetate
CoA transferase
Second
mechanism

BIOMEDICAL
IMPORTANCE
Fatty acids
are broken down in mitochondria by oxidation to acetyl-CoA in a process
that generates large amounts of energy.
When this
pathway is proceeding at a high rate, three compounds,
-
acetoacetate,
-
D-3hydroxybutyrate,
and
-
acetone,
known
collectively as the ketone bodies, are produced by the liver.
Acetoacetate
and D-3-hydroxybutyrate are used as fuels by extrahepatic tissues in normal
metabolism, but overproduction of ketone bodies causes ketosis.
Increased
fatty acid oxidation and consequently ketosis is a characteristic of starvation
and of diabetes mellitus.
Since ketone
bodies are acidic, when they are produced in excess over long periods, as in
diabetes, they cause ketoacidosis, which is ultimately fatal.
Because
gluconeogenesis is dependent on fatty acid oxidation, any impairment in fatty
acid oxidation leads to hypoglycemia.
This occurs
in various states of carnitine deficiency or deficiency of essential enzymes
in fatty acid oxidation, for example, carnitine palmitoyltransferase, or
inhibition of fatty acid oxidation by poisons, for example, hypoglycin.
The
acetyl-CoA formed in β-oxidation is oxidized in the citric acid cycle, or it
enters the pathway of ketogenesis to form ketone bodies.
As the level
of serum FFA is raised, proportionately more FFA is converted to ketone bodies
and less is oxidized via the citric acid cycle to CO2.
A fall in
the concentration of oxaloacetate, particularly within the mitochondria, can
impair the ability of the citric acid cycle to metabolize acetyl-CoA and divert
fatty acid oxidation toward ketogenesis.
Such a fall
may occur because of an increase in the NADH/NAD+ ratio caused by increased
β-oxidation of fatty acids affecting the equilibrium between oxaloacetate
and malate, leading to a decrease in the concentration of oxaloacetate,
and when
gluconeogenesis is elevated, which occurs when blood glucose levels are low.
The activation
of pyruvate carboxylase, which catalyzes the conversion of pyruvate to
oxaloacetate partially alleviates this problem, but in conditions such as
starvation and untreated diabetes mellitus, ketone bodies are overproduced
causing ketosis.
Concentration of Ketone Bodies
• Concentration of total ketone bodies in
the blood of wellfed individuals does not normally exceed 0.2 mmol/L (10-30
mg/L)
• Urine: Loss via urine is usually less
than 1 mg/day in humans.
02. Mechanism of ketosis in diabetes
mellitus and starvation. Ketoacidosis.
Ketoacidosis Results from Prolonged Ketosis Higher
than normal quantities of ketone bodies present in the blood or urine
constitute ketonemia (hyperketonemia) or ketonuria, respectively.
The overall
condition is called ketosis.
The basic
form of ketosis occurs in starvation and involves depletion of available
carbohydrate coupled with mobilization of FFA.
This general
pattern of metabolism is exaggerated to produce the pathologic
states found in diabetes mellitus,
-
the
type 2 form of which is increasingly common in Western countries;
-
twin
lamb disease; and
-
ketosis
in lactating cattle.
Nonpathologic
forms of ketosis are
found under conditions of
-
high-fat feeding and
-
after
severe exercise in the postabsorptive state.
Acetoacetic
and 3-hydroxybutyric acids are both moderately strong acids and are buffered
when present in blood or other tissues.
However,
their continual excretion in quantity progressively depletes the alkali
reserve, causing ketoacidosis.
This may be
fatal in uncontrolled diabetes mellitus.
03. Biosynthesis of fatty acids:
3.1. Sources of acetyl
CoA and NADPH I cytoplasm.
3.2. Synthesis of malonyl
CoA.
3.3. Fatty acids
synthesis, structure.
3.4. Biosynthesis of
palmitic acid: reactions.
There are
few systems for fatty acid synthesis.
01.Extramitochondrial system: responsible for de novo synthesis of palmitic
acid (always end product) from acetyl-CoA(start).
-
(De Novo Synthesis)
-
The
synthesis takes place in cytosol.
02. Chain Elongation Systems:
a) Microsomal: present in microsomes which can lengthen
existing fatty acid chains.
b) Mitochondrial: this system is mostly restricted
to lengthening of an existing fatty acid of moderate chain-length.
It operates under anaerobiosis
and is favored by a high NADH/NAD+ ratio.
Materials Required for the Synthesis
01. Enzymes
–
Acetyl-CoA
carboxylase,
–
Fatty
acid synthase, a multienzyme complex
02. Coenzymes
and cofactors:
-
Biotin,
NADPH, Mn++
03. CO2
04. ATP
3.1. Sources of acetyl
CoA and NADPH I cytoplasm.
Sources of acetyl-CoA
Acetyl-CoA
is mainly found in mitochondria and cannot pass out.
It forms citrate
by condensing with oxaloacetate.
Citrate is
transported out.
Once in
cytoplasm, an enzyme citrate lyase cleaves citrate to form acetyl-CoA and
oxaloacetate.
Carnitine-acetyl transferase may probably transfer acetyl group of acetyl-CoA to carnitine to form acetylcarnitine in mitochondria.
After translocation to cytoplasm acetyl group may be transferred to CoA to make it acetyl-CoA.
Sources of NADPH
Pentose
phosphate pathway is
the main source of NADPH.
Cytoplasmic
enzyme called malic enzyme (NADP-malate dehydrogenase) catalyzed the
reaction in which malate is oxidatively decarboxylated to pyruvate and NADPH
is produced.
Cytoplasmic isocitrate dehydrogenase uses NADP as the coenzyme.
3.2. Synthesis of malonyl
CoA.
Reaction occurs in two steps:
01. Biotin-enzyme + ATP + HCO3 –
02.Carboxy-biotin-enzyme + Acetyl-CoA
Malonyl CoA + biotin-enzyme
The
reaction is irreversible.
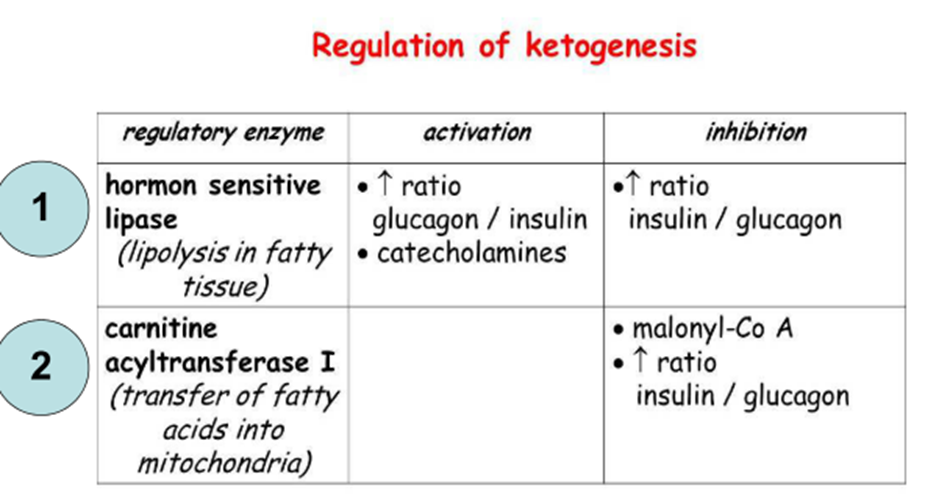
Regulation
Acetyl-CoA
carboxylase catalyzes
the rate limiting step in the de novo synthesis of fatty acids and
provides the earliest point at which control can be exerted.
The enzyme
is inactivated by phosphorylation.
A: Insulin, CoA, Guanine
nucleotides
I: Glucagon, adrenaline, acyl-CoA Decrease in
citrate concentration decreases acetyl-CoA carboxylase activity
3.3. Fatty acids synthase,
structure.
It is a multienzyme
complex.
It is made
up of an ellipsoid dimer of two identical polypeptide monomeric units,
arranged in
a “head to tail” fashion
The ACP has an –SH group in the 4phosphopantothene moiety, referred as pantothenyl-SH (Pan-SH)
Another
active –SH group present in the cysteine moiety of the enzyme ketoacyl synthase, referred as cysteinyl-SH (Cys–SH).
The “Pan-SH”
of one monomeric unit is in close proximity to the “Cys-SH” group of other
monomeric unit and vice-versa.
Sequence of domaines in primary structure of fatty acid syhthase monomer
-
ketoacyl
synthase,
-
malonyl/acetyl
transacylase,
-
hydratase
-
enoyl
reductase,
-
ketoacyl
reductase,
-
ACP
-
thio-esterase
(deacylase)
Complex is
functional only when the two monomeric units are in association
with each other.
The
functional activity is lost when they are dissociated.
In a dimer
form, the complex jointly synthesises 2 molecules of palmitic acid
simultaneously.
Sequence
of enzyme domains in primary structure of fatty acid synthase monomer
3.4. Biosynthesis of palmitic acid: reactions.
4. Biosynthesis of triacylglycerols.
TAG (neutral
fats) is the main form of energy deposition.
Deposited
fat can provide the body with energy during fasting for a long time (up
to 7-8 weeks).
TAG
synthesis occurs in absorptive period in the liver and adipose tissue.
-
However,
if the adipose tissue participates only in fat deposition,
-
the
liver plays an important role in converting carbohydrates originating
from food in fats which are then secreted into the blood as part of VLDL
and delivered to other tissues.
The
immediate substrates for the synthesis of fats are the acyl- CoA and glycerol-3-phosphate.
The
metabolic pathway of synthesis of fats in the liver and adipose tissue
is the same,
-
except
for the different pathways of glycerol-3-phosphate
synthesis.
The liver
is the main organ where synthesis of fatty acids from the products of glycolysis
takes place.
In the
smooth endoplasmic reticulum of hepatocytes fatty acids, interacting
with glycerol-3phosphate, are activated and immediately used for the
synthesis of TAG.
Synthesised
fats are packaged in VLDL and secreted into the blood.
In adipose
tissue for TAG synthesis mainly fatty acids released by the hydrolysis
of ChM and VLDL fats, are used.
Fatty acids
come into adipocytes, where they are transformed into derivatives
of CoA and react with glycerol-3-phosphate.
Furthermore,
in these cells synthesis of fatty acids from products of glycolysis occurs.
TAG
molecules in adipocytes are combined into larger oil droplets,
containing no water, which is the most compact form of fuel storage
molecules.
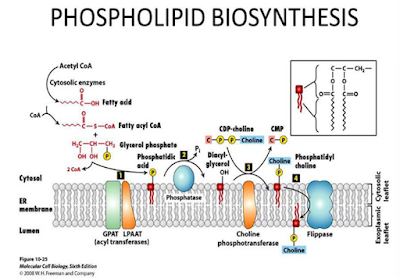
5. Biosynthesis of phospholipids.
Phospholipids
Phospholipids
are a specialized group of lipids performing a variety of functions.
These
include the membrane structure & functions involvement in blood clotting &
supply of
arachidonic acid for
the synthesis of prostaglandins.
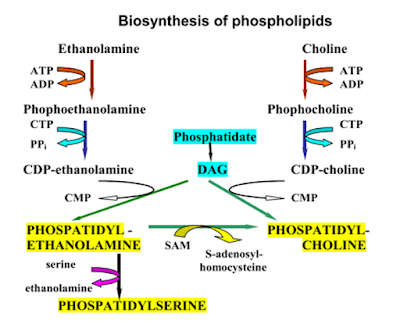
Synthesis of phospholipids
Phospholipids
are synthesized from phosphatidic acid & 1.2-diacylglycerol,
intermediates
in the production of triacylglycerols.
Phospholipid’s
synthesis occurs in the smooth endoplasmic reticulum.
Inner
mitochondrial membrane
Formation of lecithin & cephalin
It occurs
mainly in liver & brain.
Choline
& ethanolamine first
get phosphorylated & then combine with CTP to form CDP-choline
& CDP-ethanolamine.
Phosphatidylcholine (lecithin) is synthesized when CDP-choline
combines with 1,2-diacylglycerol.
Synthesis of phosphatidylserine
Phosphatidyl
ethanolamine can
exchange its ethanolamine group with free serine to produce phosphatidylserine.
Formation of phosphatidylinositol
CDP-diacylglycerol produced from phosphatidic acid
combines with inositol to form phosphatidyl
inositol (PI).
Phosphatidyl
inositol contains arachidonic acid on carbon 2of glycerol which serves as a
substrate for prostaglandin synthesis.
Pl is important for signal transmission across membranes.
The
synthesis of phospholipids requires
• glycerol
• fatty
acids
• inorganic
phosphates
• nitrogen
bases (in particular, choline for synthesis of phosphatidylcholine)
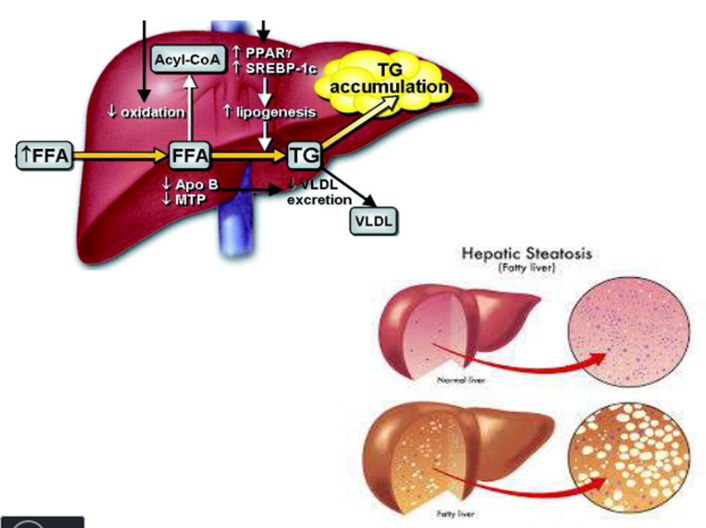
6. Fatty infiltration of liver.
Lipotropic agents.
-
In
insufficient synthesis of choline, or its short supply to
the liver,
-
the
synthesis of phospholipids from neutral fat components becomes either
impossible, or drastically decreased,
which
results in deposition of neutral fats in the liver.
Such
condition is referred to as fatty infiltration of
liver,
which may
subsequently develop into a fatty degeneration of the liver (steatosis)
In other
words, the synthesis of phospholipids needs either choline or compounds
capable to act as methyl group donors and thus participate in the production
of choline (for example, methionine)
Such
compounds are known as lipotropic agents
curd cheese is
recommended in the diet as lipotropic agent,
since its
ingredient is casein, a protein whose molecule contains a large number
of methionine residues























Comments
Post a Comment