BIOCHEMISTRY OF LIVER
z CLASS № 33
THEME:
BIOCHEMISTRY OF THE LIVER
1. Role of
the liver in carbohydrate, lipid, amino acid and protein metabolism.
2.
Detoxification functions of the liver.
3. Heme
synthesis, reactions.
4.
Degradation of heme. Bilirubin metabolism, scheme.
5. Disorders
in bilirubin metabolism: jaundice, its types.
6.
Biochemical mechanisms of hepatic failure and hepatic coma. Biochemical tests
for diagnosis of liver disorders.
Liver is…
• The largest organ in the body
• Weighs 1.2 to 2 kg
• Constitutes about 2-3% of body weight.
• Involved in many digestive, vascular, and metabolic
activities.
• Has over
500 vital functions.
MAJOR FUNCTIONS OF THE LIVER
I.
Homeostatic function.
–
processing
all of the gastrointestinal blood through the portal vein.
–
regulation
the blood levels of glucose, amino acids, and other nutrients taken from food.
II.
Biosynthetic function.
Production
and secretion of compounds for extrahepatic tissues (most blood proteins,
coagulation factors, lipids, glucose, ketone bodies, etc.).
III.
Storage function.
Place for
storage of glycogen, Fe, trace elements, vitamins (retinol, A, D, K, folic
acid, B12).
III.
Protective (detoxification) function.
-
Transformation
of harmful substances (such as ammonia and toxins) into less harmful compounds.
- Metabolism of most hormones, and ingested drugs to water soluble products for excretion.
-
Metabolism
of ethanol. Ex.: Kupffer cells in the liver ingest bacteria or other foreign
material from the blood.
V.
Digestive function.
Synthesis of
bile acids and production/secretion of the bile.
VI.
Excretory function.
Excretion of
various substances with the bile (water, cholesterol, bile pigments,
phospholipids, bicarbonate and other ions).
VII. Metabolic
function.
The central
role in metabolism of most nutrients taken from food (carbohydrates, lipids,
proteins, amino acids, porphyrins, etc.)
1. Role of the liver in carbohydrate,
lipid, amino acid and protein metabolism.
01. Carbohydrate metabolism in the liver
Regulation
of the blood glucose level.
Oxidative
degradation of
glucose either to CO2 and H2O, or lactate,
Synthesis of
glycogen, conversion of glycogen to glucose,
Gluconeogenesis, or synthesis of glucose from
non-carbohydrate compounds,
Conversion
of glucose via pentose phosphate pathway,
Conversion
of dietary monosaccharides, e.g. fructose and galactose to glucose.
Metabolism
of glucose to glucoronic acid.
1. During intensive exercises skeletal muscles produce
lactate from the glucose taken from the blood (anaerobic glycolysis).
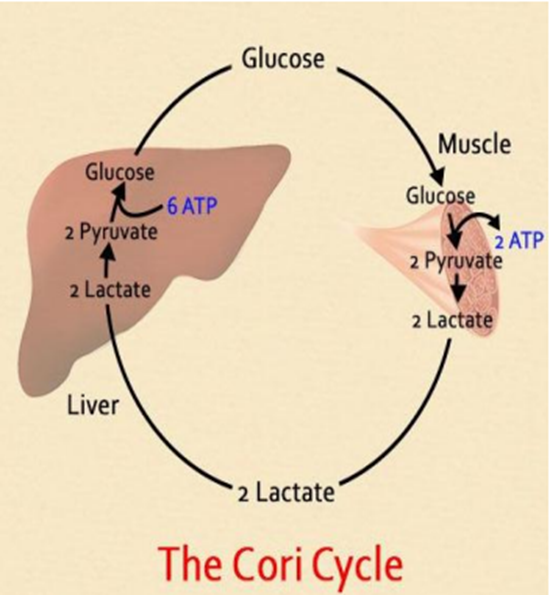
3. The glucose is taken back to the muscles and used for production of energy
On average, the adult liver stores about 80 g of glycogen,
and in the fasting state releases 9 g of
glucose each hour to the blood to maintain the peripheral glucose
concentration.
The contribution from gluconeogenesis increases
progressively with fasting as glycogen stores become further depleted at the
rate of 11% per hour.
The carbon substrates for gluconeogenesis are derived from
both lactate released by glycolysis in the peripheral tissues and from hepatic
deamination of amino acids from the proteolysis of skeletal muscle.
Energy for gluconeogenesis comes from the β-oxidation of
fatty acids.
The end product of this process, acetyl-CoA, also stimulates
the activity of the first committed enzyme of gluconeogenesis, pyruvate
carboxylase.
02. Lipid metabolism in the liver
1) Uptake, b-oxidation of free fatty acids for energy.
2) Synthesis of triacylglycerols, phospholipids,
cholesterol, and esters of cholesterol.
3) Metabolism of plasma lipoproteins (VLDL, HDL,
chylomicron remnants).
4) Ketone body synthesis.
5) Metabolism of phospholipids.
6) Synthesis of bile acids.
7) Hydroxylation of the vitamin D.
Lipoprotein metabolism in the liver
• Triacylglycerols, phospholipids and cholesterol produced in
the liver are packed into lipoproteins VLDL and HDL.
• The liver is the place for synthesis of apoproteins and
enzymes for lipoprotein metabolism.
• Destruction of cholesterol-rich HDL and chylomicron
“remnants”.
The liver synthesizes fatty acids from acetate units.
The fatty acids formed are then used to synthesize fats and
phospholipids, which are released into the blood in the form of lipoproteins.
The liver’s special ability to convert fatty acids into
ketone bodies and to release these again is also important.
The liver is the major site of both synthesis and catabolism
of cholesterol, which is transported to other tissues as a component of
lipoproteins.
Excess cholesterol is converted into bile acids in the liver
or directly excreted with the bile. Bile acids are key elements in fat
metabolism.
Bile acids have a detergent-like effect, solubilizing
biliary lipids and emulsifying dietary fat in the gut to facilitate its
digestion.
They are synthesized by hepatocytes.
03. Protein metabolism in the liver
1. Synthesis
of proteins and enzymes for own use.
2. Synthesis
of apo-proteins and enzymes for lipoprotein metabolism.
3.
Production and secretion of the most of plasma proteins:
–
albumin,
–
ceruloplasmin,
–
transferrin,
–
clotting
factors (fibrinogen, prothrombin, coagulation factors V, VII, IX, X, and XI),
– acute-phase proteins (C-reactive protein, haptoglobin, etc.).
Amino acid metabolism in the liver
1)
Maintenance of the plasma amino acid pool.
2) Catabolism
of amino acids taken from blood stream: (deamination, transamination,
transdeamination, etc.).
3) Synthesis
of urea, detoxification of ammonia (convertion of α-ketoglutarate into
glutamate).
4) Synthesis
of creatine.
5) Formation of uric acid from purine bases.
2. Detoxification
functions of the liver.
DETOXIFICATION and DRUG METABOLISM
•
Detoxification reactions include conversion of toxic, nonpolar compounds to
the less toxic and more readily extractable compounds.
• In
detoxification the toxicity may be either completely eliminated, or lessened.
• The liver
metabolizes most of
–
exogenous substances (xenobiotics, drugs, ethanol),
–
endogenous substances (steroid hormones, bilirubin, etc.).
Phase of detoxification in the liver
• Phase I:
involves a
super family of CYP monooxygenases.
In these
reactions the substance polarity increases by hydroxylation catalyzed by
microsomal cytochrome P450 oxidases (microsomal
oxidation).
• Phase II:
cytoplasmic
enzymes conjugate
the functional groups introduced in the first phase reactions, by glucuronidation,
or other reactions.
Microsomal oxidation
• takes
place in the endoplasmic reticulum (microsomes),
• conducts
to hydroxylation of a non-polar substance R-H into the polar water-soluble
product R-OH.
The process
requires O2, NADPH(H+), a flavoprotein enzyme, and cytochrome P450.
R-H + O2 + NADPH + H+ → R-ОH + H2O + NADP+
Conjugation reactions
I.
Glucuronidation.
Involves
conjugation of a substance with glucuronic acid using UDP-glucuronic acid
and a family of UDP-glucuronyl transferases.
• Ex. -
Production of direct bilirubin from indirect bilirubin).
2. Conjugation with glycine
• Glycine is
conjugated with such compounds as benzoic acid, nicotinic acid,
para-aminobenzoic acid, etc.
• Hippuric
acid is produced after introduction of sodium benzoate to the body.
The rate of
synthesis and renal excretion of hippuric acid is measured to test the
detoxification ability of the liver.
Sulfatation
• involves a
sulfotransferase enzyme catalyzing the transfer of a sulfo group (-SO3
) from 3'- phosphoadenosine-5'-phosphosulfate (PAPS), to a substrate
molecule's hydroxyl or amine.
• Ex.:
detoxification of indole and skatole that are toxic derivatives of tryptophan
formed in bacterial putrefaction.
Other detoxification reactions
01. Acetylation reactions
are used in detoxification of xenobiotics, and sulfonamide drugs.
X +
AcetylCoA = Acetyl-X + CoA (where X is a xenobiotic)
02. Methylation reactions. (methylation of
xenobiotics, pyridine and nicotinic acid with use of S-adenosine methionine).
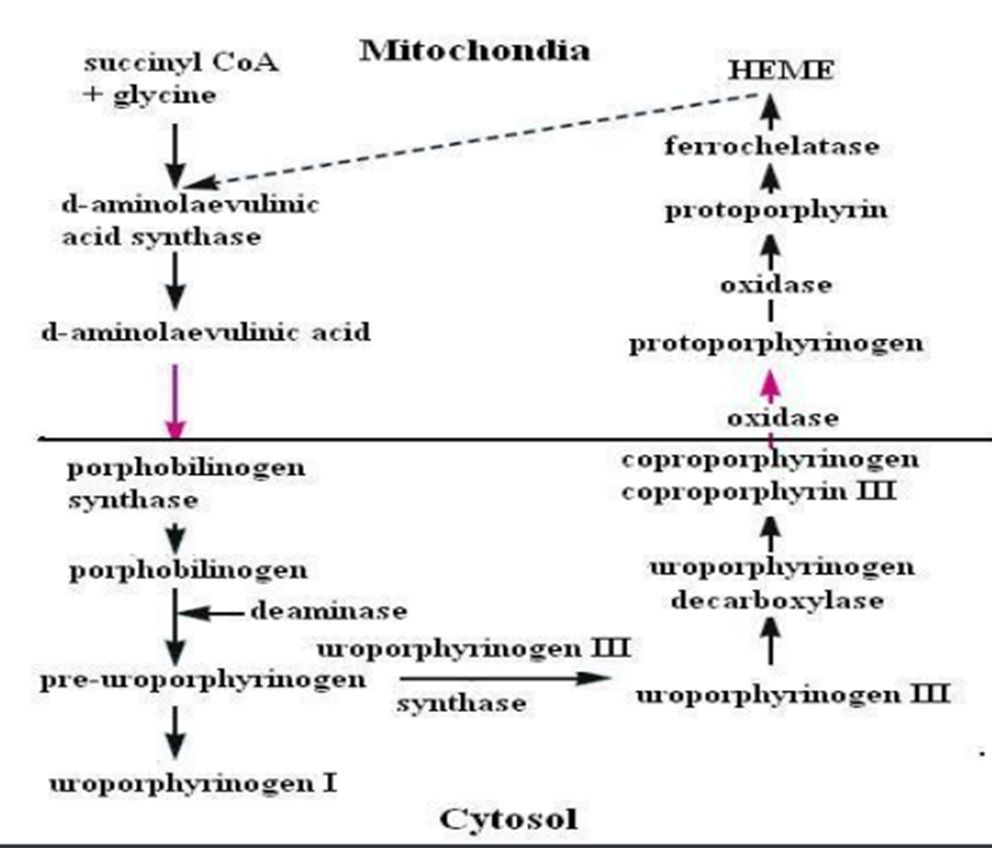
3. Heme synthesis,
reactions.
–
hemoglobin,
myoglobin,
–
catalases.
• Synthesis
of heme mainly takes place in
–
bone
marrow (80 – 85%)
–
liver
(15%).
• 300 mg
of heme is produced daily in the body, of which only 1% is excreted unused
in the urine and stools.
• 1/3 of the
heme produced in the liver is required for the formation of cytochrome P
450.
• Heme
synthesis begins in the mitochondria with condensation of
succinyl-CoA with glycine to form δaminolaevulinic acid.
The reaction
in catalyzed by δaminolaevulinic acid synthase, which is the most
crucial enzyme.
BILIRUBIN METABOLISM
• Bilirubin
is the orange-yellow pigment derived from senescent red blood cells;
• It is a toxic
waste product in the body;
• It is
extracted and biotransformed mainly in the liver, and excreted in the bile
and urine;
• It is a bile
pigment;
• Elevations
in serum and urine bilirubin levels are normally associated with Jaundice.
•
Determination of the levels of total bilirubin, indirect and direct bilirubin,
bile pigments is used for diagnosis of liver diseases.
• Metabolism of bilirubin takes place in the cells of reticuloendotelial system, the liver and intestine.
Formation of indirect bilirubin in the reticuloendothelial system
Indirect bilirubin
• In RES
cells the ring structure of the heme is opened and the iron atom is removed by
the action of heme oxygenase and cytochrome P450 to produce the green-colored
intermediate biliverdin.
• Biliverdin
is reduced by biliverdin reductase to form indirect bilirubin.
• Indirect
(or unconjugated) bilirubin is a toxic, water-insoluble substance.
It is bound to serum albumin and transported to the sinusoidal membrane of the liver cells as a bilirubinalbumin complex.
Conjugation of indirect bilirubin in the liver
• In the
liver, bilirubin is conjugated to two molecules of glucuronic acid to form
bilirubin diglucuronide (or direct, conjugated bilirubin).
• Conjugated
bilirubin is water-soluble, less toxic substance, which is subsequently
eliminated via the bile and urine.
Conjugated
form of bilirubin is normally present in the blood in 3 – 10% of the
total serum bilirubin.
• The liver
secretes conjugated bilirubin into the biliary canaliculi and finally to the
small intestine
Conversion of bilirubin to other bile pigments
i.
In
the intestine, conjugated bilirubin is converted back to unconjugated
bilirubin by bacterial β-glucuronidases in the distal ileum and
colon;
ii.
After that unconjugated bilirubin is reduced to the colored bile
pigments: mesobilinogen, urobilinogen, and stercobilinogen.
iii.
Up
to 80% of urobilinogen produced daily is reduced to stercobilinogen.
iv.
The
rest 20% of urobilinogen is reabsorbed from the intestine and enters the
enterohepatic circulation. The liver breaks down about 5 % of this urobilinogen
to di- and tri-pyrrole compounds, which are excreted in the urine. The rest of
the reabsorbed urobilinogen comes back to the intestine with the bile.
v.
In
the colon, the stercobilinogen spontaneously oxidized to stercobilin (otherwise
known as fecal urobilin), which is colored; most stercobilin is excreted in the
feces, and is responsible for the color of feces.
vi.
A
small fraction of stercobilinogen, (2 % – 5 %) enters the general circulation
and appears in the urine.
Reference ranges of bile pigments in biological fluids
–
Total
bilirubin – 5.0 – 20.5 mkmol/l (blood);
–
Indirect
bilirubin – 1.7 – 17.1 mkmol/l (blood);
–
Direct
bilirubin – 1.0 – 7.5 mkmol/l (blood);
–
Stercobilinogen
– 4 mg/day (urine);
–
Stercobilin
– 250 mg/day (feces).
5. Disorders in
bilirubin metabolism: jaundice, its types.
Jaundice
• Hyperbilirubinemia
is elevated level of total bilirubin, resulted from imbalance between its
production and excretion.
• Jaundice
becomes clinically evident when the serum bilirubin level exceeds 27 - 34
mkmol/l.
Symptoms:
–
Icterus, or
yellow discoloration of the skin, sclerae, and mucous membrane.
–
Itching due to
deposits of bile salts on the skin;
–
Changes
in the color of stool.
–
Deep orange and foamy urine.
Different causes of jaundice
–
Excessive
Production of Bilirubin
–
Reduced
Hepatocyte Uptake
–
Impaired
Bilirubin conjugation
–
Impaired
Bile Flow
Classification
I.
Prehepatic (hemolytic)
II.
Intrahepatic (hepatocellular)
III.
Posthepatic (obstructive)
Prehepatic (hemolytic) Jaundice
• Results
from excess production of bilirubin (beyond the livers ability to
conjugate it) following hemolysis.
Blood:
–
Increased
indirect bilirubin
–
Unchanged
direct bilirubin.
Intestine:
–
overproduction
of urobilinogen and stercobilinogen from unconjugated bilirubin.
Urine:
–
increased
level of stercobilinogen.
Stool:
– Dark brown stool, markedly increased stercobilin.
Intrahepatic (hepatocellular) jaundice
• impaired hepatic
uptake, conjugation, or secretion of bilirubin
• reflects low
conjugation efficiency of hepatocytes resulted from liver disease,
either inherited or acquired (hepatitis, etc.) .
Blood:
– both indirect and direct bilirubin increased
Intestine: – Conjugated bilirubin is not
efficiently secreted into the bile. Low production of stercobilinogen.
Urine:
– Deep yellow because of excreted direct bilirubin. – Appearance of
urobilinogen (impaired reduction of urobilinogen to the di- and tri-pyrrol end
products).
Stool:
– Reduced stercobilin, Pale coloured stool
Posthepatic (obstructive) jaundice
• Results
from obstruction of the bile flow between the liver and intestine caused by
structural disorders of the bile duct, tumors in the bile duct,
cholelithiasis.
Blood:
– Increased direct bilirubin – Unchanged indirect bilirubin.
Intestine: – Very low production of urobilinogen and stercobilinogen.
Urine:
– Dark colored urine because of excretion of direct bilirubin.
Stool:
– Clay colored stool. Absence of stercobilin.
High bilirubin in neonates
• Neonates
are especially vulnerable to high unconjugated bilirubin levels due to
an immature blood-brain barrier that predisposes them to kernicterus/bilirubin
encephalopathy (bilirubin accumulates particularly in the basal nuclei),
which can
result in permanent neurological damage with seizures, abnormal reflexes
and eye movements etc.
• Neonates
also have a low amount of functional UDP-glucuronyl-transferase and can
have elevated unconjugated bilirubin, since conjugated is limited.
• Neonates
in general are at increased risk since they lack the intestinal bacteria that
facilitate the breakdown and excretion of conjugated bilirubin in the feces
(this is largely why the feces of a neonate are paler than those of an adult).
Instead the conjugated bilirubin is converted back into the unconjugated form by the enzyme β-glucuronidase (in the gut, this enzyme is located in the brush border of the lining intestinal cells) and a large proportion is reabsorbed through the enterohepatic circulation.
6. Biochemical
mechanisms of hepatic failure and hepatic coma. Biochemical tests for diagnosis
of liver disorders.
Hepatic failure and hepatic coma
• Liver
failure or hepatic failure is the inability of the liver to perform its
normal synthetic and metabolic function.
• Two forms
are recognized, acute and chronic liver failure.
Acute liver failure
• Is the
rapid development of hepatocellular dysfunction,
specifically
coagulopathy and mental status changes (encephalopathy) in a patient
without known prior liver disease.
•
Hepatocellular disease may ALTER PROTEIN SYNTHESIS in the liver,
especially plasma albumin, and coagulation factors II, VII, IX, and X.
• The diagnosis of acute liver failure is based on physical exam, laboratory findings, patient history, and past medical history to establish mental status changes, coagulopathy, rapidity of onset, and absence of known prior liver disease respectively
Chronic hepatic failure
• usually occurs
in the cirrhosis as the result of many possible causes, such as
excessive alcohol intake, hepatitis B or C, autoimmune, hereditary and
metabolic causes (iron or copper overload, steatohepatitis or nonalcoholic
fatty liver disease.
• Main
causes of the liver failure:
–
Acute
viral hepatitis;
–
Cirrhosis
(alcoholic or non-alcoholic);
–
Excessive
injuries or traumas;
–
Sepsis;
– Poisonings by hepatotrophic venoms and medicines.
Clinical findings in liver failure
–
Hyperbilirubinemia;
–
Low
total serum protein and albumin level;
–
Coagulopathy
and hemorrhage because of impaired synthesis of clotting proteins;
–
Low
levels of potassium, sodium, and calcium in the blood;
– High levels of toxic phenol and indol derivatives, aromatic, branched and sulfur-containing amino acids in the blood.
Hepatic coma
is a state
of unconsciousness which the patient cannot be aroused, even by powerful
stimuli.
Hepatic coma
accompanies cerebral damage resulting from degeneration of liver
cells especially that associated with cirrhosis of the liver.
Liver functional tests
• are groups
of blood tests that provide information about the state of a patient's liver.
A panel of
biochemical measurements is routinely performed in the clinical laboratories on
plasma or serum specimens.
• The
standard liver panel includes determination of:
–
Total
serum protein and albumin (low plasma albumin is detected in acute and chronic
liver diseases);
–
Total
bilirubin, direct and indirect bilirubin, other bile pigments;
–
Blood
ammonia (elevated in cirrhosis of the liver and disorders of the urea cycle);
–
Alanine
aminotransferase (AlAT), aspartate aminotransferase (AsAT) (higher increases in
the AlAT activity compared to the AsAT activity);
–
Alkaline
phosphatase (ALP) (increases in cholestasis);
–
Gamma-glutamyl
transferase (GTT) (increases in alcohol abuse and hepatitis).
Other liver tests
Coagulation
test.
–
prothrombin
time
–
prothrombin
ratio (PR)
–
international
normalized ration (INR);
Determination
of ceruloplasmin, serum glucose, cholesterol, urea.
Determination
of alpha-fetoprotein (AFP) (increases in hepatocelular carcinoma)
Lactate
dehydrogenase (LDH4 and LDH5)













Comments
Post a Comment